Hematopoietic stem cell transplantation
Hematopoietic stem cell transplantation (HSCT) is a treatment that involves suppressing a patient’s haematopoietic system by chemotherapy or radiotherapy and replacing it either with stem cells previously harvested from this patient or with cells from another individual (donor). One of the benefits of this treatment is that it lets one use more aggressive treatment in patients with resistant tumors. In addition, the transplanted cells themselves may have a curative effect on the patient’s haematological malignancies [1, 2].
Traditionally haematopoietic stem cells have been harvested from bone marrow. Nowadays, peripheral blood enriched with mobilized stem cells is used several times more frequently than bone marrow [4]. Peripheral blood has several advantages over bone marrow as a source of stem cells. Collecting haematopoietic stem cells from peripheral blood is less invasive than sourcing them from bone marrow and does not require anesthesia. It is predicted that peripheral blood will continue to be the major source of haematopoietic stem cells for HSCT in the future.
Apheresis is a procedure required to collect stem cells from peripheral blood and is a part of peripheral blood stem cell transplantation. The stem cell transplantation process can be summarized in distinct phases such as mobilization, apheresis (collection), preparing the product for storage, infusing the transplant, and engraftment and recovery.
XN Stem Cells: The technology behind automated stem cell counting
Automated stem cell counting is a very simple, reliable and standardized method. 190 μL of blood or apheresis material is aspirated and the result is available in a few minutes with no need for manual gating, pre-treatment or sample washing. Four separate stem cell counts are performed from the 190 μL aliquot, after which the mean value of the four measurements is reported, making the analysis particularly accurate and reliable. Stem cell counts are reported as an absolute (XNHPC#) and a relative count (XN-HPC% – a percentage of the total white blood cells).XN Stem Cells are enumerated on the XN haematology analyser using fluorescence flow cytometry. The instrument uses a channel dedicated to white precursor and pathological cells, called ‘WPC’. During analysis, signals of forward scattered light, sideward scattered light and side fluorescence light are recorded. Forward scatter (FSC) corresponds to the size of the cell and side scatter (SSC) to the internal structural complexity/granularity. The fluorescence intensity (SFL) depends on several factors, such as the cell’s state of maturity and whether it is reactive or malignant in origin [7]. In the WPC channel, the fluorescence intensity of cells depends on the cells’ permeability to the WPC reagent. A high degree of membrane damage by the reagent leads to cellular content leaking through the pores, a decreased cell size, and higher fluorescence intensity since more fluorescence marker can enter the cell and bind to the DNA [8].
Immature stem cells differ from the more mature progenitor cells by their membrane lipid composition. Stem cells’ membranes are relatively resistant to permeabilisation by the WPC reagent [8]. As a result, stem cells are medium in size (medium FSC), have a low granularity (low SSC) and relatively low fluorescence intensity (low-medium SFL). Based on fluorescence flow cytometry, the analyser uses three dimensions to identify stem cells (see Fig. 1). Other cell populations, such as NRBC, myeloid progenitor cells or lymphocytes, which look similar to stem cells from a morphological perspective, do not interfere with the stem cell population since they have a different membrane composition. This result in a very different cell volume and fluorescence intensity in the WPC channel compared to stem cells. As a result, there is good stem cell separation and count value accuracy.
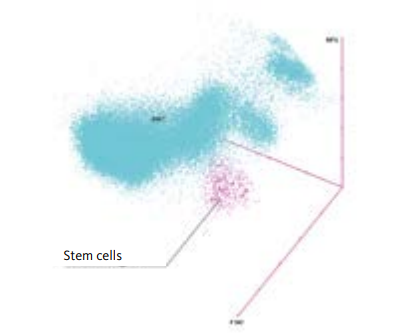 Fig 1. XN Stem Cells cluster in the 3D model of a WPC channel scattergram
Fig 1. XN Stem Cells cluster in the 3D model of a WPC channel scattergram
Optimizing the current stem cell apheresis workflow
XN Stem Cells is a solution that you can use to optimize the stem cell apheresis workflow in the following stages:
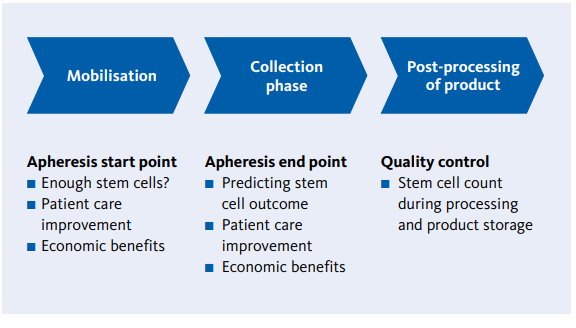 Fig. 2 Schematic overview of implementation points for XN Stem Cells to optimise stem cell apheresis
Fig. 2 Schematic overview of implementation points for XN Stem Cells to optimise stem cell apheresis
Mobilisation and enumerating CD34
In healthy donors, the percentage of CD34+ cells circulating amongst the total nucleated cell population in peripheral blood in a steady state is approximately 0.06 %, or 2 – 5/μL. However, the mobilisation of stem cells from bone marrow into peripheral blood prior to stem cell collection increases the concentration of stem cells by a factor of 10 – 100 [6]. The optimal concentration of stem cells for successful engraftment is 5 – 10 x 10^6 CD34+ cells/kg of recipient body weight. The minimum concentration is 2 x 10^6 CD34+ cells/kg body weight. Historically, an increase in the WBC count has been seen as a marker for stem cell mobilisation.However, it is reported that a WBC count correlates only weakly with the CD34+ count in mobilised peripheral blood [9].
CD34+ cells are enumerated in mobilised peripheral blood using immune flow cytometry according to the suggested ISHAGE protocol [10]. In general, commercially available kits for staining CD34, CD45 and apoptosis/cell viability marker 7AAD are used. On average, the CD34 count in mobilised blood is performed two to three times in autologous transplantations, and once in allogeneic transplantations on the donor blood. Even though CD34 flow cytometry is the current ‘gold standard’ for stem cell evaluation, the method has some limitations.
Firstly, the procedure is lengthy. The sample requires labeling and several washing steps, and the full analysis procedure may take up to 1.5 hours. Secondly, the commercial antibody kits are rather expensive. Thirdly, correct gating of the cell populations on the flow cytometer requires experienced laboratory personnel, and results may vary significantly depending on the flow cytometer’s operator. Other factors that affect the variation of the counts within and between the laboratories include the type of flow cytometer, the protocol used, and the antibody manufacturer [11]. Finally, external quality control schemes showed a high variation in CD34 counts between laboratories [12], and there are reported cases when anti-CD34 antibodies interacted with substances in the plasma and caused false-positive CD34 analysis results [13].
The XN Stem Cells method has shown excellent correlation with the CD34 count in mobilised peripheral blood [14 – 16]. The table below (Table 1) summarizes the correlations between XN Stem Cells and CD34 in mobilised peripheral blood and apheresis products observed in the latest multicenter study in the Netherlands.
Table 1. Correlations of XN Stem Cells and CD34 in mobilised peripheral blood and apheresis samples (Gromme et.al.,submitted manuscript)
| |
Slope |
Intercept, cells/μL |
r value |
Number of samples |
| All samples |
0.95 |
0.52 |
0.97 |
214 |
| Peripheral blood |
0.92 |
0.98 |
0.92 |
147 |
| Intermediate apheresis product |
0.86 |
146.1 |
0.98 |
22 |
| Final apheresis product |
0.99 |
92.07 |
0.94 |
45 |
XN Stem Cells can be used to substitute most of the CD34 counts in mobilised peripheral blood to determine the starting point of stem cell collection. Studies have shown that identical cutoff values should be used for CD34 and XN Stem Cells counts [15]. It was recommended to use a higher cut-off value (38 cells /μL) for good mobilisers and a lower cut-off value (20 cells /μL) for expected poor mobilisers. Sysmex recommends that customers evaluate which cut-off value best suits their routine as it will depend on the range of pathologies in their transplantation centre. A cut-off value for XN Stem Cells lower than 20 cells /μL should not be used. As a starting point, CD34 counts should be performed in the mobilised blood sample with an XN Stem Cells count above, but close to, the cut-off value of 20 cells /μL.
The following cut-off values could be used as guidance yet should still be evaluated at the site:
- XN-HPC < 20/μL ➞ continue mobilisation regimen; do not perform CD34count; patient will return the next day
- XN-HPC ≥ 20 and < 30/μL ➞ perform CD34 test; start or postpone apheresis based on the CD34 count according to the laboratory SOP
- XN-HPC ≥ 30/μL ➞ Start apheresis; CD34 test may be omitted
Stem cell collection
Mobilised stem cells are collected from peripheral blood during stem cell apheresis using a blood cell separator. An apheresis procedure requires several hours, usually four to Five, stem cells continue to be mobilised from bone marrow into peripheral blood during apheresis, and up to 55 % of the stem cells in the final. Apheresis product represents those mobilised during the apheresis procedure itself. Both the drop in stem cell concentration and the mobilisation during apheresis vary substantially among individuals [17, 18].
Apheresis centres use a formula to calculate the required volume of apheresis and to predict the stem cell yield, based on the CD34 count pre-apheresis in the mobilised blood, the expected efficiency of apheresis and the weight of the patient [19].During a multi centre study in the Netherlands, the XN Stem Cells count from an intermediate apheresis product showed a good correlation (r = 0.98) with the CD34 yield in the final apheresis product (Gromme et al., submitted manuscript).
Apheresis product post-processing
Some guidelines require a CD34 count to be performed in the final product [10]. In some hospitals, however, stem cell collection, post-processing of the product, storage and the transplantation itself is carried out at different locations. Customer surveys have shown that particular apheresis centres are interested in a quick and easy stem cell count in the final product before sending the product away for post-processing and storage. XN Stem Cells is such a quick and reliable method for an additional stem cell count on the apheresis product.
Benefits of XN Stem Cells for managing stem cell apheresis – a case study
XN Stem Cells is a simple, quick and affordable method for enumerating stem cells that is easy to standardize and implement at any stem cell transplantation centre. It leads to savings in terms of time and costs thanks to an optimized apheresis workflow. The benefits of apheresis workflow management can be illustrated through a case study of transplantation centre in Germany. The centre performs 80 transplantations per year, all of which are autologous. Three CD34 measurements are performed prior to stem cell collection to determine the apheresis starting point. There is no monitoring of apheresis during stem cell collection, and CD34 is counted one final time in the apheresis product. Around 20 % of patients are very poor mobilisers and have to return for the second round of apheresis on the next day if the yield of CD34+ cells in the final product is low. For these patients, an additional CD34 count is performed the next morning prior to stem cell collection. After implementing XN Stem Cells, two of the initial CD34 analyses from mobilised peripheral blood are omitted. XN Stem Cells are counted starting from day three or four of the mobilisation protocol. As soon as the XN Stem Cells count reaches the cut-off value of 20 cells /μL, CD34 analysis is performed and if the CD34+ count is also above the cut-off value stem cell collection is started.
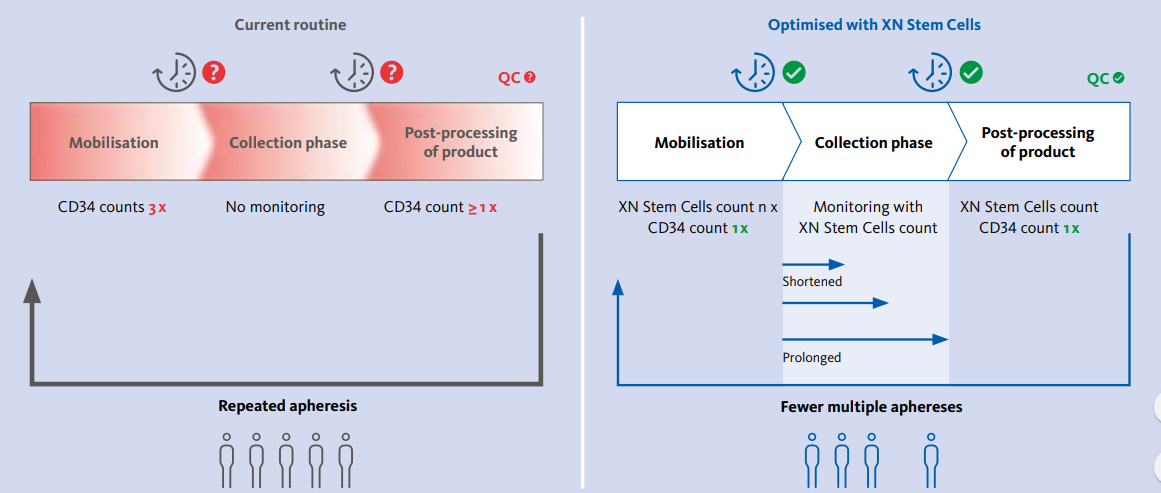 Fig 3. Schematic representation of the apheresis workflow before and after implementing XN Stem Cells
Fig 3. Schematic representation of the apheresis workflow before and after implementing XN Stem Cells
One additional XN Stem Cells analysis is performed after two cycles of apheresis to assess the stem cell collection efficiency. This lets the centre identify patients with low collection efficiency. In such cases, apheresis can be prolonged on the first day of collection. As a result, some of these patients do not have to return for collection the next day. Alternatively, if the identified collection efficiency is higher than predicted, apheresis can be stopped earlier if the target yield of stem cells has already been collected after two cycles (Fig. 3). This helps to manage beds in the apheresis ward and leads to the following benefits:
- Increased efficiency in the laboratory due to a reduced number of CD34 tests.
- Higher turnaround of patients in the apheresis ward thanks to an improved apheresis workflow.
- Cost reduction thanks to the lower number of CD34 tests.
- Improved patient experience for patients who can avoid repeated apheresis
Conclusion
XN Stem Cells is a novel method for enumerating stem cells based on fluorescence flow cytometry. The analysis is fast, simple and standardized. Moreover, it requires no extra instrument as it is run on the laboratory’s routine haematology analyser. Enumerating stem cells from mobilised peripheral blood and intermediate apheresis products can substantially improve the apheresis workflow in terms of saving both time and costs.
Literature:
- Copelan EA et al. (2006): Hematopoietic stem-cell transplantation. N Engl J Med. 354(17) : p. 1813
- Hatzimichael E et al. (2010): Hematopoietic stem cell transplantation. Stem Cells Cloning. 3 : p. 105 – 17.
- Worldwide Network for Blood and Marrow Transplantation (WBMT) (2013): 1 millionth blood stem cell transplant marks major medical milestone. Bern, Switzerland, 2013.
- Gratwohl A et al. (2010): Hematopoietic stem cell transplantation: A global perspective. JAMA. 303(16) : p. 1617 – 24.
- Passweg J R et al. (2016): Hematopoietic stem cell transplantation in Europe 2014: More than 40 000 transplants annually. Bone Marrow Transplant. 51(6) : p. 786 – 92.
- Korbling M et al. (2001): Peripheral blood stem cell versus bone marrow allotransplantation: Does the source of hematopoietic stem cells matter Blood. 98(10) : p. 2900 – 8.
- Schuff-Werner P et al. (2016): Performance of the XN-2000 WPC channel-flagging to differentiate reactive and neoplastic leukocytosis.Clin Chem Lab Med. 1 ;54(9) : 1503 – 10.
- Kawauchi S et al. (2013): The Positions of Normal Leukocytes on the Scattergram of the Newly Developed Abnormal Cell-detection Channel of the XN-Series Multi-parameter Automated Hematology Analyzers. Sysmex Journal International. Vol.23 No.1.
- Yu J et al. (1999): The predictive value of white cell or CD34+ cell count in the peripheral blood for timing apheresis and maximizing yield. Transfusion. 39(5) : p. 442 – 50
- Barnett D et al. (1999): Guideline for the flow cytometric enumeration of CD34+ haematopoietic stem cells. Prepared by the CD34+ haematopoietic stem cell working party. General Haematology Task Force of the British Committee for Standards in Haematology. Clin Lab Haematol. 21(5) : p. 301 – 8.
- Levering WH et al. (2007): Flow cytometric CD34+ stem cell enumeration: Lessons from nine years’ external quality assessment within the Benelux countries. Cytometry B Clin Cytom. 72(3) : p. 178 – 88.
- Moroff G et al. (2006): Multiple-laboratory comparison of in vitro assays utilized to characterize hematopoietic cells in cord blood. Transfusion. 46(4) : p. 507 – 15.
- Liwski CJ et al. (2014): Discordant CD34+ cell results in peripheral blood and hematopoietic progenitor cell-apheresis product: Implications for clinical decisions and impact on patient treatment. Transfusion. 54(3) : p. 541 – 4.
- Park SH et al. (2015): The new Sysmex XN-2000 automated blood cell analyzer more accurately measures the absolute number and the proportion of hematopoietic stem and progenitor cells than XE2100 when compared to flow cytometric enumeration of CD34+ cells. Ann Lab Med. 35(1) : p. 146 – 8
- Peerschke EI et al. (2015): Evaluation of new automated hematopoietic progenitor cell analysis in the clinical management of peripheral blood stem cell collections. Transfusion. 55(8) : p. 2001 – 9.
- Tanosaki R et al. (2014): Novel and rapid enumeration method of peripheral blood stem cells using automated hematology analyzer. Int J Lab Hematol. 36(5) : p. 521 – 30.
- Hester J et al. (2000): Peripheral blood stem cell collection: The inter- action of technology, procedure, and biological factors. Transfus Sci.23(2) : p. 125 – 32.
- Humpe A et al. (2013): Successful mobilization, intra-apheresis recruitment, and harvest of hematopoietic progenitor cells by addition of plerixafor and subsequent large-volume leukapheresis. Transfus Med Hemother. 40(4) : p. 251 – 7.
- Hosing C et al. (2014): Peripheral blood stem cell yield calculated using preapheresis absolute CD34+ cell count, peripheral blood volume processed, and donor body weight accurately predicts actual yield at multiple centers. Transfusion. 54(4) : p. 1081 – 7.
References:
- Edited from White_Paper/Haematology/Sysmex_White_Paper_Management_of_Stem_Cell_Apheresis.pdf