India has the third largest HIV epidemic in the world. In 2017, HIV prevalence among adults (aged 15-49) was an estimated 0.2%. This figure is small, compared to most other developing countries but because of India's huge population, it equates to 2.1 million people living with HIV. Overall, India’s HIV epidemic is slowing down. Between 2010 and 2017, new infections declined by 27% and AIDS-related deaths more than halved, falling by 56%.
This welcome decline is majorly attributed to the growing awareness of the HIV transmission and subsequent complications, more efficient treatment centers, and better diagnostic techniques that help in early detection and improved management of HIV infection.
Like most other infectious diseases, HIV diagnosis is made by either demonstrating the presence of virus or viral products in the host, alternatively by detecting host response to the virus. An HIV diagnosis is commonly made through serological assays to detect HIV specific antibodies or HIV antigen, or by Nucleic Acid Amplification Test (NAAT) to detect HIV nucleic acids.
Following HIV transmission, markers of infection can be detected by diagnostic assays in a predictable fashion. An ‘eclipse phase’ typically lasting 10–14 days is characterized by infection in local mucosal and lympho-reticular tissue during which systemic viremia is not detected. Approximately 7 days later, viral RNA becomes present in serum at quantities detectable by NAAT. Capsid protein p24 is the next chronological measurable component of acute infection by way of detection of soluble antigen. Approximately 5 days after detection of p24 antigen, seroconversion occurs and anti-HIV antibody becomes evident by reactive results to ELISA assays.
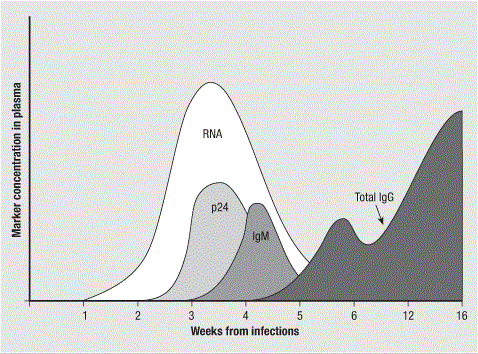 Virological and serological markers during the first weeks following infection with HIV-I. Data indicated in this figure was previously reported from Murphy G. and Parry J.V and published no Eurosurveillance. Vol.13, Issue 7-9 (Jul-Sep 2008) (Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18966)
Virological and serological markers during the first weeks following infection with HIV-I. Data indicated in this figure was previously reported from Murphy G. and Parry J.V and published no Eurosurveillance. Vol.13, Issue 7-9 (Jul-Sep 2008) (Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18966)
Due to technical improvements and new developments of immunological assays, the reliability of serological laboratory diagnosis of HIV infection has improved considerably and the residual risk, due to the diagnostic window for transfusion-transmitted HIV, has been reduced significantly. As an alternative to NAAT, combined fourth-generation assays have been developed and have achieved a high degree of sensitivity and specificity. A combination, or 4th generation assay, is designed to detect both the p24 antigen and HIV antibodies in a single test. Combination tests can detect HIV as early as 2–6 weeks after infection, and are recommended in laboratory testing.
Fourth generation tests can effectively shorten the time frame in which HIV infection is undetectable prior to seroconversion. While reported sensitivity and specificity of fourth generation tests remain higher than 99%, in numerous studies, several instances of discordance between screening and confirmatory techniques have been recorded.
Reported reasons of cross-reactivity in HIV ELISAs.:
- Cross-reactive antibodies to some human leukocyte antigens (HLA) that develop as the result of prior blood transfusion
- Multiple pregnancies
- Auto-antibodies found in some rheumatologic diseases
- Heating of serum
- Freeze-thaw of serum
- Recent vaccination (influenza, hepatitis)
- Renal transplantation
- Some liver diseases
- Malignancy
- EBV infection
- Positive RPR tests (syphilis, autoimmune diseases)
- Technical errors in testing
Various concurrent clinical conditions including viral hepatitis, infection with Mycobacterium tuberculosis, and recent Rickettsia infection, have been similarly associated with false positive screening tests in some studies.
The rate of unspecific reactivities is slightly higher with third-generation assays.
It is important to shorten the window period after acute HIV infection in which infected individuals are still antibody-negative, especially in blood donors. Transasia Bio-Medicals' ErbaLisa HIV Gen 4, a fourth generation ELISA for the early detection of HIV infection uses purified recombinant HIV antigens and monoclonal anti-p24 antibodies for the specific detection of HIV-1 & 2 antibodies and p24 antigen. Documented specificity of 99.8%, sensitivity of 100% and an analytical sensitivity of 8 IU/ml for p24 antigen, ensures minimum cross-reactivity and reliable detection of HIV infection.
References:
- https://www.avert.org/professionals/hiv-around-world/asia-pacific/india
- Screening of HIV infection: role of molecular and immunological assays. Weber B. Expert Rev Mol Diagn. 2006 May; 6(3):399-411.
- https://en.wikipedia.org/wiki/Diagnosis_of_HIV/AIDS
- http://www.uphs.upenn.edu
- Spectrum of false positivity for the fourth generation human immunodeficiency virus diagnostic tests. Peter Liu, Patrick Jackson, Nathan Shaw and Scott Heysell. AIDS Res Ther (2016) 13:1.
- A new combined HIV p24 antigen and anti-HIV-1/2/O screening assay. Polywka S, Duttmann H, Lübben F, Laufs R, Feldner J. Methods Mol Biol. 2005; 304:229-43.