- Authors : Dr. Mohit Vijay Rojekar, Dr. Vandana Kumavat, Dr. Jayesh Panot , Dr. Arati Adhe Rojekar, Dr. Poonam Lalla
- Conducted at: Dept. Of Biochemistry, Rajiv Gandhi Medical College & Chatrapati Shivaji Maharaj Hospital, Kalwa, Mumbai, Maharashtra.
Click to know more: http://www.mdpub.net/?mno=132810
Introduction:
Dengue is a highly prevalent mosquito-borne disease caused by Aedes aegypti mosquitoes that act as a vector for the transmission. Dengue is marked by symptoms like dengue hemorrhagic fever (DHF), dengue septic shock (DSS), skin rash, nausea, vomiting, abdominal pain and hepatosplenomegaly.[1,2] Due to the severity of the disease, it is highly important to diagnose dengue early and accurately. Serum ferritin and lipid profile tests are widely used for diagnosis and prognosis of the disease. [3, 4]
In dengue fever, serum ferritin (FT) value excessively in- creases as compared to any other bacterial or viral disease. The FT level >50 ng/ml defined as hyperferritinaemia.[5] FT is ex- pressed by the reticuloendothelial system in response to the inflammation and infection, and hyperferritinemia is an ideal prognostic marker for severity of dengue infection.[6] As ferritin binds to iron, regulates its dispersal in the circulatory system, the iron is the primary requirement of pathogenic microorganisms for their proliferation. Hence, the hyperferritinemia indicates highly active dengue infection, causing coagulation distress and enhanced immunological performance of lymphocytes, neutrophils, and macrophage serum ferritin level is disproportionately high and indicates the increased risk of developing the complications. This is also supported by the reports of high serum ferritin correlation with severity of dengue infection. [3, 7, 8]
Dengue immunopathogenesis leads to an upsurge of cytokines, infection-enhancing antibodies, inflammatory cells and ultimately organ dysfunctions and death. Dengue infection alters the lipid metabolism of the host during its life cycle. Hypolipidaemia is an independent predictor of the severity of dengue infection in critical patients. Dengue virus (DNGV) synthesis viral organelles in the host endoplasmic reticulum recognized as a replication complex (RCs). The RCs is made up of lipids like fatty phospholipids, fatty acids and cholesterol. For this activity, non-structural protein 3 (NS3) utilizes the fatty acid synthase (FASN) and 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) for an increase in the cholesterol uptake with the help of RCs. This process results in the formation of triacylglycerol after DNGV infection. DNGV also involved in the degradation of the host lipid droplets. The low-density lipoprotein receptor (LDLr) is an ideal marker for identification of the alterations in lipid metabolism and diseases severity during the viral infection. However, few studies contradict this statement due to lack of consistency in the association of DNGV infection and lipid levels. [9, 10]
Currently, unavailability of antiviral therapy is a challenge in the treatment of DNGV infection. Hence, it is essential to have a reliable prognostic biomarker to predict the severity of DNGV infection and disease management. A study claimed that high serum ferritin could be used as a predictive marker for disease severity (days of hospitalization) in the acute phase of infection. [3] This is because the source of ferritin is macrophages which are the primary targets in the dengue virus. It reported that activated macrophages contribute to the severity of dengue virus infection. High ferritin levels suggest the active disease status with hyper activation of the immune system and coagulation failure. Therefore, the present study aimed to investigate the correlation of serum ferritin, platelets and lipid profile with disease severity and if these could serve as a predictive marker for progression to severe disease.
Material and Methods:
This cross-sectional, observational study was approved by the Institutional Ethics Committee (EC/376/2019). It was conducted during June 2019 to January 2020 in the tertiary care hospital affiliated to a medical college in western India. Before enrolment, as per Helsinki declaration of 1975, written informed consent was obtained from the parents/legal guardians of the patients. The necessary sample size was 46 (for the case and control each) based on sample size calculation using 2-sided 95% confidence interval and 80% power along with the mean and standard deviation (SD). Mean ± SD for platelets of the pilot study conducted for cases was 2.64 ± 0.82, and control was 3.13 ± 0.84. Therefore, a total of 50 subjects were enrolled in each group, considering 10% dropouts. Fifty Cases (mean age of 8.7 ± 0.37) were selected randomly using ‘Random Number Generator Software’ to avoid the selection bias among the patients visiting the hospital during the study period. Patients with NS1 positivity (Days 2–8) and/or positive IgM for dengue (Days 6-10) from the date of onset of fever were included in the study as dengue cases. The study patients included were those who had a fever, hepatomegaly or splenomegaly, bicytopenia (Total leucocyte count 500 ng/ml and triglyceride level >150 mg/dl. [11, 12].
Patients on steroids or immuno-suppressant with known autoimmune disorders were excluded. Children <1 year of age, due to the possible presence of maternal antibodies, children displaying signs of altered consciousness at the time of recruitment, as well as children with nephrotic syndrome or obesity (body mass index 32), were also excluded. Another 50 patients (mean age of 8.92 ± 0.41) selected randomly, of known diseases presenting with short term fever (lower respiratory tract / upper respiratory tract infections) within seven days of onset. In these patients, the diagnosis of dengue was excluded by establishing other confirmed diagnosis.
The clinical and biochemical parameters like the history of the present febrile illness, patient demographics, presence of WHO warning signs for severe dengue[¹³] associated co-morbidities and length of hospital stay used to analyze the patients. Complete blood count at the time of admission and 48 hours interval along with Ferritin by Immunoturbidimetric assay [15], renal profile, liver function tests and lipid profile was conducted.
Serum Ferritin levels were measured if the presentation was less than seven days from the onset of fever. After written in- formed consent, blood was collected from the study subjects; Ferritin and lipid profile assays were done on XL-640 Biochemistry FAA manufactured by Transasia Biomedicals (Erba-Manheim) at the time of diagnosis if the diagnosis was made at the study centre, or at the time of admission if referred from an outside hospital with positive test results.
Statistical analysis:
Normality of the distribution of the patients’ data was assessed by the Shapiro-Wilk test. Two sample t-tests were applied for hypothesis testing. The statistical significance level was set at
There were no differences between the two groups with regards to age and sex. The results obtained were analyzed using appropriate statistical software. Descriptive statistics were reported as mean ± Standard deviation (SD), median with a coefficient of variation. Mann Whitney U test was used to com- pare platelet count and serum ferritin between case and control groups. Spearman’s rank correlation was used to assess the correlation between age with platelet count, lipid profile, serum ferritin and hospital stays. A P-value of less than 5% was considered statistically significant.
Results:
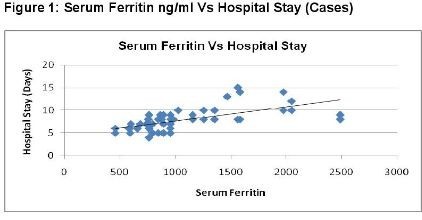
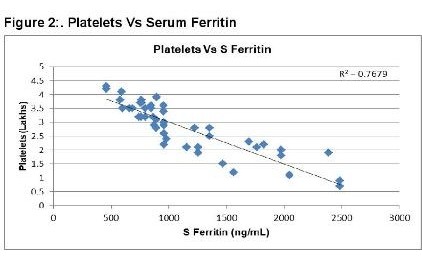
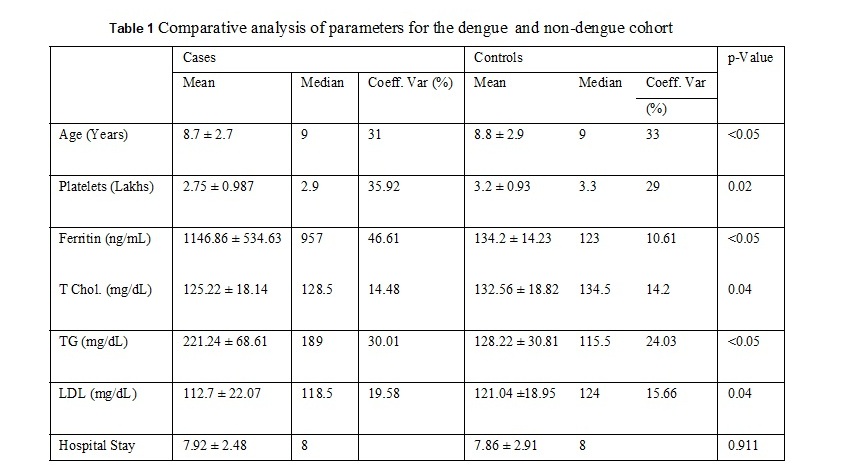
A hundred individuals (47 males and 53 females) were selected for the study in which 50 individuals had severe DNGV infection, and 50 individuals have infection other than DNGV. The mean age of the patient was 8.8 ± 2.8 years. The average hospital stay for the patients was 7.9 ± 2.5 days. The values of different parameters, including lipid profile among the dengue and non- dengue cohort, are as enlisted in Table 1. As shown in figure 1, serum Ferritin showed a strong positive correlation with a hospital stay for the cases. A higher value of Ferritin increased the hospital stay for dengue patients. This shows increased Ferritin has some role either in the etiology or is the result of dengue infection. Serum Ferritin has shown a very poor correlation with the age of the patient, as in Figure 2. As shown in figure 3, serum Ferritin has a very strong negative correlation with platelet counts in cases (R = -0.875).
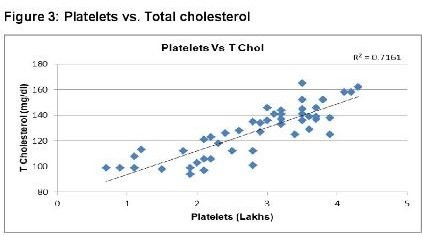
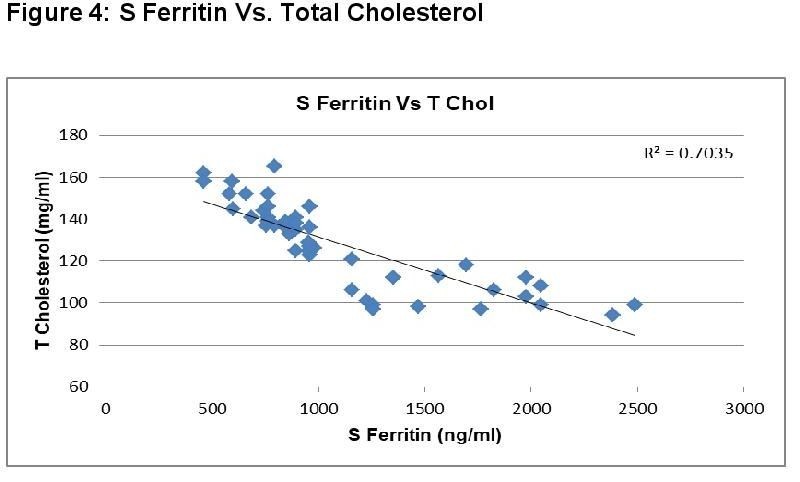
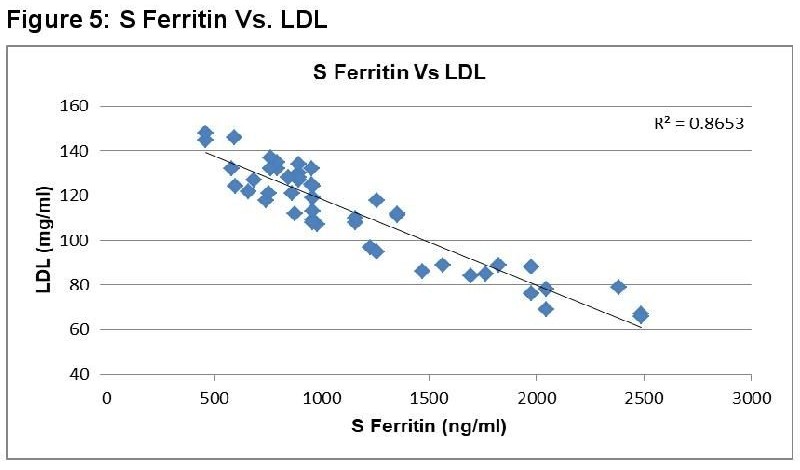
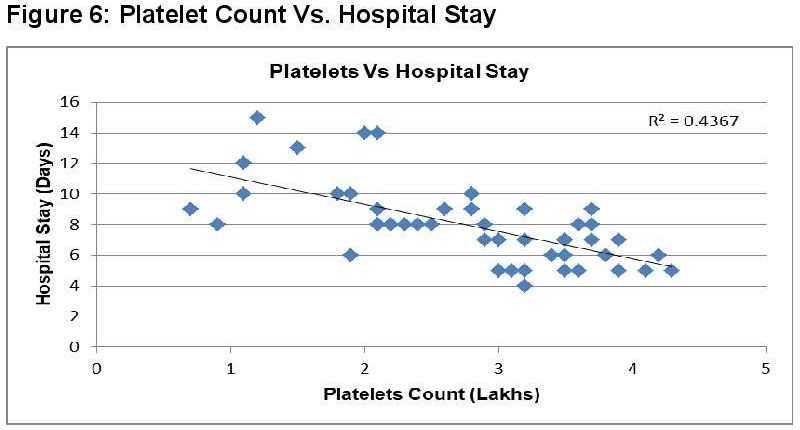
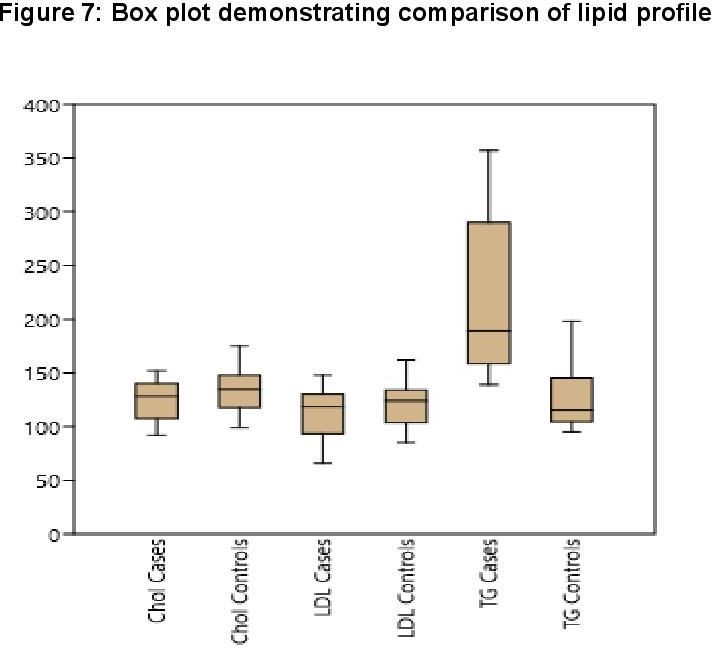
In dengue cases as Ferritin increases, platelet count decreases. This is an ominous sign in dengue infection prognosis. Similar correlation (figure 6) has been observed in cases among serum Ferritin and serum LDL with correlation coefficient -0.930. Serum cholesterol has strong positive and negative correlation with Platelets (figure 4, R = 0.846) and serum Ferritin (figure 5, R = 0.838) respectively. Figure 7 shows the correlation between the platelet count and hospital stay, which is a strong negative. As platelet count increases, a good prognostic marker, hospital stay duration decreases. In Figure 8 shown is the box plot distribution of triglycerides, total cholesterol and low density lipoproteins. Box plots demonstrate 95% CI for comparison between lipid profile of cases and controls. Mean TG levels were higher in cases than controls (p-value < 0.05). Mean LDL and cholesterol levels were lower in cases than controls. (P-value = 0.04).
Discussion:
There is the availability of diagnostic marker, but no perfect prognostic biomarker is available for monitoring the severity of DNGV infection. So it is necessary to have prognostics biomarker like ferritin or lipid profile for the DNGV infection.[3, 13] The present study analyzed the ability of ferritin and lipid profile in the prognosis of DNGV infection severity. The inclusion of patients with mild fever with another origin than DNGV infection shows a different approach than the earlier published studies. Serum ferritin level is reported to be elevated in various other infections like hepatitis C, H5N1 and West Nile Virus (WNV) infection. The present study showed a significant increase in serum ferritin level in DNGV infected patients. TG level was found to be directly proportional to DNGV infection and LDL level as inversely proportional to the DNGV infection. Lima WG et al. (2019as documented influence of factor like ethical or racial difference, age and study pattern on the association of lipid profile with DNGV infection.[10] Survarna et al. (2009) has documented the role of lipid profile as unclear DNGV infection and focused on a protective role of lipoproteins in the immunopathogenesis of DNGV infection.[9]
Hyperferritinaemia is the most important component of the MAS (Macrophage activation syndrome). Elevated Ferritin is supposed to have two different functions in the dengue fever. In the initial phase of the disease, Ferritin acts as protective in nature by chelating toxic free iron radicals at the site of inflammation. In severe cases, raised ferritin seems to be pathogenic in nature by activating immune cells which results in cytokine storm. [3, 15]
Results of the present study showed that hospital stay du- ration increased with an increase in serum Ferritin level Mean hospital stay for the patients was 7.9 ± 2.5 days, and ferritin (p<0.05). More the serum ferritin levels more is the time required by the patient to recover. Thus, serum ferritin can be considered as an indicator of the severity of illness. Serum ferritin level can be used to predict the course of the disease or prognosis. Meaning if the patient is screened for the serum ferritin, these levels can guide us about the future course of the patient. Based on ferritin levels, patients need careful monitoring to avoid complications. [7] Similar results were obtained in a study conducted in the children where it was observed that increased serum ferritin was associated and well correlated with the severity of dengue. [3, 11, 6] A similar study from Indian subcontinent also correlated the raised serum ferritin with the severity of the disease. [17] This shows that serum ferritin can be used as an early marker of the illness and its severity.
Conclusion:
The present study had shown an excellent correlation of high serum ferritin levels and the severity of dengue. However, the relationship between lipid profile as a whole or its components and the severity and/or prognosis of the dengue needs further research and assessment.
Conflict of Interest:
The authors have no conflicts to disclose.
Funding:
This study received no fund.
References:
- Ahmed NH, Broor S. Dengue Fever outbreak in Delhi, north India: a clinicoepidemiological study. Indian J Community Med 2015; 40(2):135–8.
- Zhang H, Zhou YP, Peng HJ, Zhang XH, Zhou FY, Liu ZH, et al. Predictive symptoms and signs of severe dengue disease for patients with dengue fever: a meta-analysis. Biomed Res Int. 2014; 359308.
- Soundravally R, Agieshkumar B, Daisy M, Sherin J, Cleetus CC. Ferritin levels predict severe dengue. Infection. 2015;43 (1):13-9.
- Chaudhuri SR, Bhattacharya S, Chakraborty M, Bhattacharjee. Serum Ferritin: A Backstage Weapon in Diagnosis of Dengue Fever. Interdiscip Perspect Infect Dis. 2017; 2017: e7463489
- Cornelia AM, Ralph MH, Pannuti CS, Brouns RM, Riemsdijk WA, Byron EE, et al. Hyperferritinaemia in Dengue Virus-Infected Patients Is Associated with Immune Activation and Coagulation Disturbances. Plos Negl Trop Dis. 2014 Oct; 8(10): e3214.
- Valero N, Mosquera J, Torres M, Duran A, Velastegui M, Reyes J, et al. Increased serum ferritin and interleukin-18 levels in children with dengue. Braz J Microbiol. 2019 Jul; 50(3): 649–656.
- Van de Weg CAM, Huits R, Pannuti CS, Brouns RM, van den Berg RWA, van den Ham HJ, et al. Hyperferritinemia in dengue virus infected patients is associated with immune activation and coagulation disturbances. Plos Negl Trop Dis. 2014;8(10):e3214
- Van de Weg C, Huits R, Brouns M, van den Berg R, Martina B, Osterhaus A et al. Elevated ferritin levels in dengue virus infected patients from Aruba. International Journal of Infectious Diseases. 2012; 16:e116.
- Suvarna JC, Rane PP. Serum lipid profile: a predictor of clinical outcome in dengue infection. Trop Med Int Health. 2009;14 (5):576-85.
- Lima WG, Souza NA, Fernandes SOA, Cardoso VN, Godói IP. Serum lipid profile as a predictor of dengue severity: A systematic review and meta-analysis. Rev Med Virol. 2019; e2056.
- Aricò M, Janka G, Fischer A, Henter JI, Blanche S, Elinder J, et al., Hemophagocytic lymphohistiocytosis. Report of 122 children from the International Registry. FHL Study Group of the Histiocyte Society. Leukemia. 1996;10(2):197
- Palazzi DL, mcclain KL, Kaplan SL. Hemophagocytic syndrome in children: an important diagnostic consideration in fever of unknown origin. Clin Infect Dis. 2003;36 (3):306.
- https://www.who.int/news-room/fact- sheets/detail/dengue-and-severe-dengue
- Roberts CH, Mongkolsapaya J, Screaton G. Dengue fever: a practical guide. Br J Hosp Med (Lond) 2012 Apr;73 (4): C60-4
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99(10):3505-16.
- Chaiyaratana W, Chuansumrit A, Atamasirikul K, Tang- nararatchakit K. Serum ferritin levels in children with dengue infection. Southeast Asian J Trop Med Public Health. 2008;39 (5):832-36.
- Ahmed A, Alvi AH, Butt A, Nawaz AA, Hanif A. Assessment of Dengue fever severity through liver function tests. J Coll Physicians Surg Pak. 2014;24 (9): 640-644.